Hepatitis C
Hepatitis C
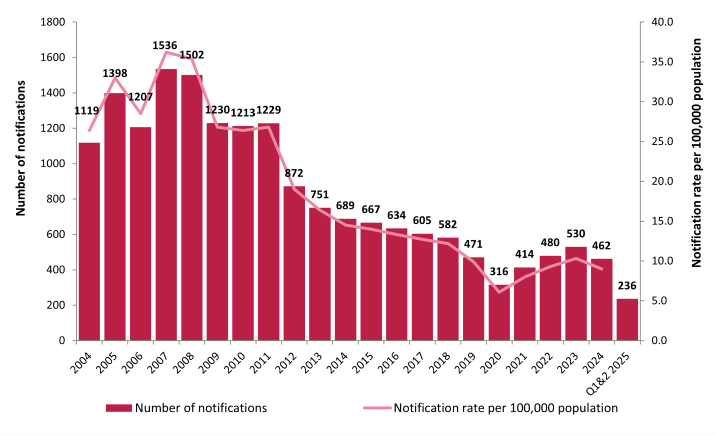
Hepatitis C is a viral infection, which causes inflammation of the liver. It is spread through contact with the blood of an infected person. Sharing injecting needles and equipment (‘works’) with someone who is infected is the most common way to get hepatitis C in Ireland. About 25-30% of people who are infected clear the virus within one year of infection. The remaining 70-75% develop chronic (long-term) infection. This can cause serious liver disease, including cirrhosis (scarring of the liver), liver cancer and liver failure. This liver damage occurs gradually over 20-30 years in people with chronic infection. Hepatitis C became a notifiable disease in Ireland in 2004.
New highly effective treatments for hepatitis C became available in Ireland in late 2014. These result in a cure for over 95% of people who are infected. For more information on treatment please see:
https://www2.hse.ie/conditions/hepatitis-c/
Last updated: 21 July 2025
Case Definitions
Hepatitis C infection (Hepatitis C virus)
Clinical criteria for acute or recent hepatitis C infection
Clinical hepatitis within the past 24 months (where other causes of acute hepatitis have been excluded) defined as peak elevated serum alanine aminotransferase (ALT) levels >200 IU/L
Laboratory criteria for acute or recent hepatitis C infection
Definitive evidence
Detection of hepatitis C antibody(anti-HCV) or hepatitis C virus RNA (HCV RNA) or hepatitis C virus antigen (HCV Ag) in a person who has had a negative anti-HCV test recorded within the past 24 months
OR
Detection of HCV RNA or detection of HCV Ag in a person with a documented negative HCV Ag/RNA test within the preceding 24 months (excluding those who are known to have been treated recently and did not achieve a sustained virological response).
OR
Detection of hepatitis C virus with a different genotype/subtype to that previously documented within the past 24 months
OR
Evidence of very recent infection: HCV RNA/HCV Ag positive AND antibody negative
Suggestive evidence
Detection of hepatitis C virus by antigen or nucleic acid testing in a person with no previous hepatitis C test results
Hepatitis C case classification
Clinical case - NA
Probable case - NA
Confirmed hepatitis C infection: acute or recent case
Definitive laboratory evidence
OR
Suggestive laboratory evidence AND clinical criteria fulfilled
Confirmed hepatitis C infection: chronic case
Does not meet criteria for acute or recent hepatitis C infection
AND
Detection of HCV RNA
OR
Detection of HCV Ag
Confirmed hepatitis C infection: unknown classification
Does not meet criteria for "Confirmed acute or recent hepatitis C infection” or "Confirmed chronic hepatitis C infection”
AND
Detection of anti-HCV confirmed by a confirmatory antibody test in persons older than 18 months without evidence of resolved infection*
*Resolved infection: Detection of hepatitis C virus antibody in a person who was also tested for hepatitis C virus RNA or core antigen and found to have an undetectable/negative result. Resolved infections should not be notified.
Last revised: 1 January 2026
Guidance

Hepatitis C Screening: National Clinical Guideline No. 15 Background supporting documents are available to view here.
- HIQA Health Technology Assessment of birth cohort testing for hepatitis C
- Guidelines for the Emergency Management of Injuries (EMI) and Post-Exposure Prophylaxis (PEP)
- Blood-Borne Viruses in the Haemodialysis, CAPD and Renal Transplantation Setting
Hepatitis C Treatment
- Public health plan for the pharmaceutical treatment of hepatitis C, 2014
- National Hepatitis C treatment programme - community treatment guidelines 2023



